English Zone
Welcome!!!
Consejos a la hora de estudiar Lecto-Comprension
1. Leer el texto completo para tener una primera impresion.
Text 1
In chemistry and physics , an atom (Greek meaning "indivisible") is the smallest possible particle of a chemical element that retains its chemical properties. The word atom may also refer to the smallest possible indivisble fundamental particle . This definition must not be confused with that of chemical atoms, since chemical atoms (hereafter "atoms") are composed of smaller subatomic particles .
2. No traducir palabra por palabra, sino oracion por oracion:
In chemistry and physics , an atom (Greek meaning "indivisible") is the smallest possible particle of a chemical element that retains its chemical properties.
3. Marcar los verbos y si es posible distinguir el sujeto del predicado:
In chemistry and physics , an atom (Greek meaning "indivisible") is the smallest
possible particle of a chemical element that retains its chemical properties.
En esta oracion el verbo es “is”. ¿En que tiempo esta? ¿Presente, pasado, futuro...?
El sujeto es “an atom (Greek meaning “indivisible”)” y el predicado es todo lo demas.
4. Marcar las palabras claves para comprender la oracion:
In chemistry and physics , an atom (Greek meaning "indivisible") is the smallest possible particle of a chemical element that retains its chemical properties.
5.Buscar en el diccionario aquellas palabras que no entiendes
6.Traducir la oracion al castellano teniendo en cuanta que hay que alterar el orden de las palabras y a veces hasta hay que agregar alguna:
En quimica y fisica, un atomo ( del griego “indivisible”) es la particulamas pequeña posible de un elemento quimico que retiene sus cualidades quimicas.
7.Releer todo el texto para asegurarse que tenga cohesion. Puede ser nesesario cambiar alguna palabra para que concuerde mejor con el significado del texto.
Atencion: ¡Para tener en cuenta!
1. No traducir palabra por palabra, recorda que el orden de las palabras cambia.
2. No es necesario buscar en el diccionario todas las palabras, solo las mas importantes. Ten en cuanta que hay frases y palabras que se pueden inferir por el contexto.
3.Cuando tengas mas de un verbo conjugado es probable que tengas una oracion dentro de otra, por ej:
“An atom is a particle and it can’t be divided.”
En esta oracion hay dos verbos: is y can’t be , aqui tenemos dos oraciones unidas por “and” cada una con su propio sujeto y predicado.
La palabra tiene significado en funsion del texto y no viceversa, ojo.
Text 1
In chemistry and physics, an atom (Greek meaning "indivisible") is the smallest possible particle of a chemical element that retains its chemical properties. The word atom may also refer to the smallest possible indivisble fundamental particle. This definition must not be confused with that of chemical atoms, since chemical atoms (hereafter "atoms") are composed of smaller subatomic particles.
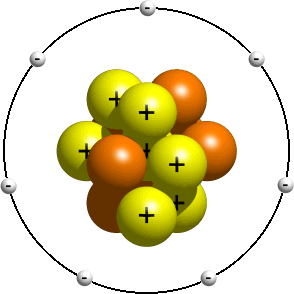
Most atoms are composed of three types of massive subatomic particles which govern their external properties:
- electrons, which have a negative charge and are the least massive of the three;
- protons, which have a positive charge and are about 1836 times more massive than electrons; and
- neutrons, which have no charge and are about 1838 times more massive than electrons.
Protons and neutrons are both nucleons and make up the dense, massive atomic nucleus. The electrons form the much larger electron cloud surrounding the nucleus.
Atoms differ in the number of each of the subatomic particles they contain. The number of protons in an atom (called the atomic number) determines the element of the atom. Within a single element, the number of neutrons may also vary, determining the isotope of that element. Atoms are electrically neutral if they have an equal number of protons and electrons. Electrons that are furthest from the nucleus may be transferred to other nearby atoms or even shared between atoms. Atoms which have either a deficit or a surplus of electrons are called ions. The number of protons and neutrons in the atomic nucleus may also change, via nuclear fusion, nuclear fission or radioactive decay.
Atoms are the fundamental building blocks of chemistry, and are conserved in chemical reactions. Atoms are able to bond into molecules and other types of chemical compounds. Molecules are made up of multiple atoms; for example, a molecule of water is a combination of two hydrogen atoms and one oxygen atom.
Text 2
History of Chemistry
The earliest practical knowledge of chemistry was concerned with metallurgy, pottery, and dyes; these crafts were developed with considerable skill, but with no understanding of the principles involved, as early as 3500 in Egypt and Mesopotamia. The basic ideas of element and compound were first formulated by the Greek philosophers during the period from 500 to 300 Opinion varied, but it was generally believed that four elements (fire, air, water, and earth) combined to form all things. Aristotle's definition of a simple body as "one into which other bodies can be decomposed and which itself is not capable of being divided" is close to the modern definition of element.
About the beginning of the Christian era in Alexandria, the ancient Egyptian industrial arts and Greek philosophical speculations were fused into a new science. The beginnings of chemistry, or alchemy, as it was first known, are mingled with occultism and magic. Interests of the period were the transmutation of base metals into gold, the imitation of precious gems, and the search for the elixir of life, thought to grant immortality. Muslim conquests in the 7th cent. diffused the remains of Hellenistic civilization to the Arab world. The first chemical treatises to become well known in Europe were Latin translations of Arabic works, made in Spain c. 1100; hence it is often erroneously supposed that chemistry originated among the Arabs. Alchemy developed extensively during the Middle Ages, cultivated largely by itinerant scholars who wandered over Europe looking for patrons.
